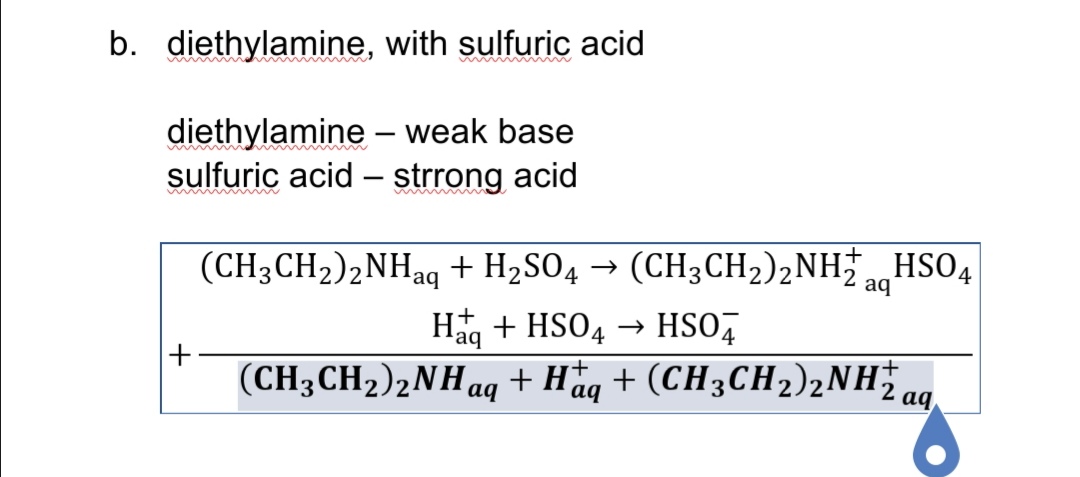
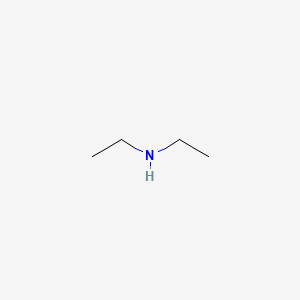
The pH of 0.05 M aqueous solution of diethyl amine is 12.0. Calculate Kb. - Sarthaks eConnect | Largest Online Education Community
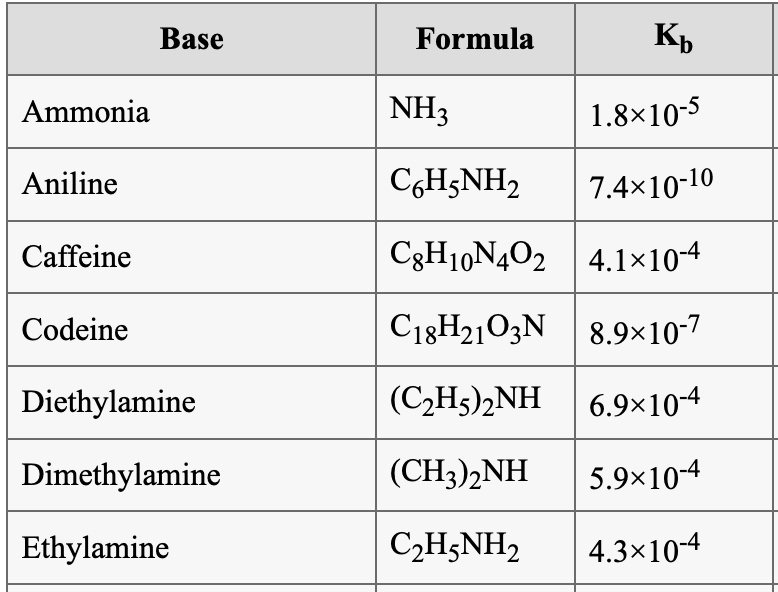
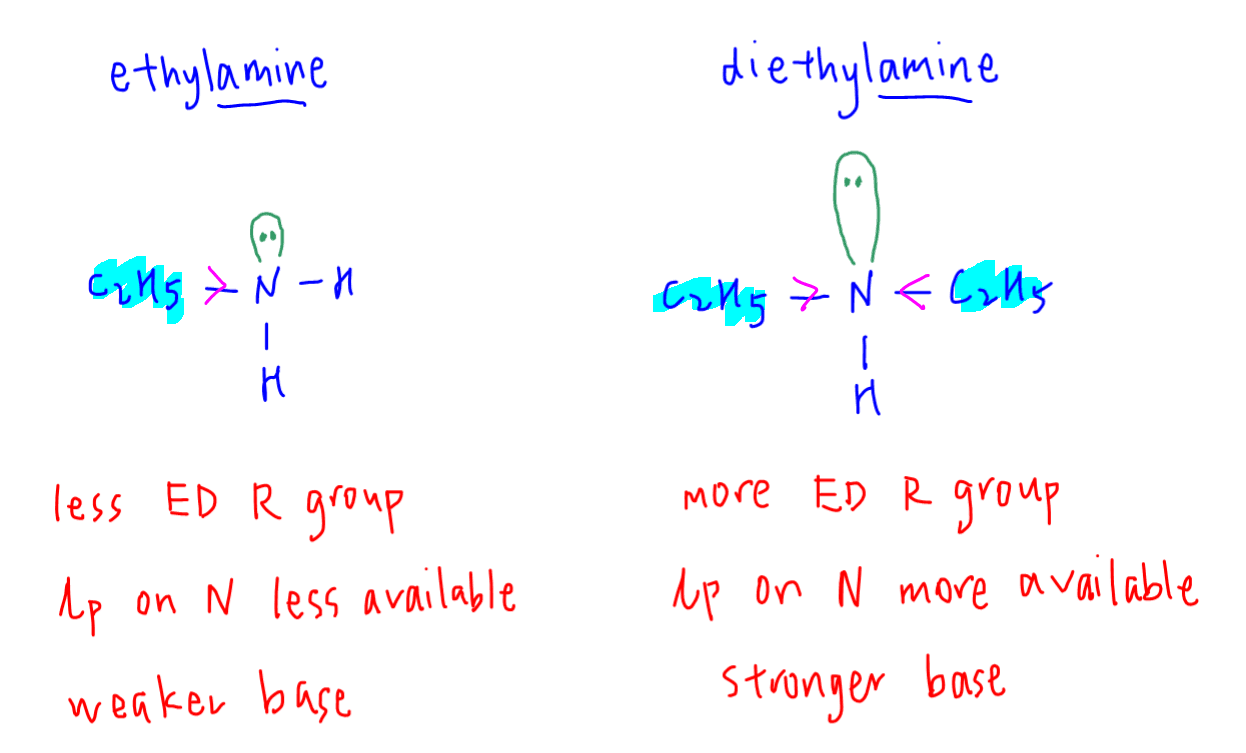
SOLVED: Base Formula Kb Ammonia NH3 Aniline C6H7N2 1.8x10^-5 7.4x10^-10 C6H10N4O2 4.1x10^4 C18H21O2N 8.9x10^-7 (C2H5)2NH 6.9x10^-4 (CH3)2NH 5.9x10^-4 C6H7N2 4.3x10^-4 Caffeine Codeine Diethylamine Dimethylamine Ethylamine

Diclofenac Diethylamine + Linseed Oil + Methyl Salicylate Gel Base Manufacturer / Supplier and Franchise